Cancer could be detected 3 years before symptoms appear with a simple blood test; new study reveals
A groundbreaking new study from Johns Hopkins University suggests that a simple blood test could detect cancer up to three years before symptoms appear. This innovative approach could revolutionize early diagnosis and significantly improve survival rates.
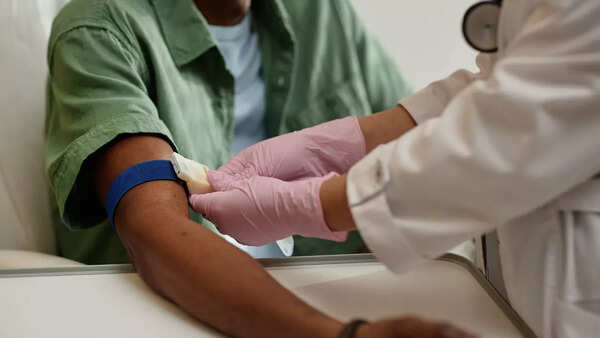
Published in the journal Cancer Discovery, the study highlights the potential of early cancer detection. Currently, late diagnosis remains a significant challenge in cancer treatment, contributing to millions of deaths annually. Early detection allows for less aggressive treatment options and significantly improves the chances of survival.
Detecting Cancer Early: A Critical Advantage
"Three years earlier provides time for intervention," explains researcher Yuxuan Wang from Johns Hopkins. Detecting tumors in their early stages means they are likely to be smaller, less aggressive, and more responsive to treatment. This advantage could be the difference between a curable and a life-threatening diagnosis.
The research centers on circulating tumor DNA (ctDNA), genetic material that tumors shed into the bloodstream. While these traces are minuscule, especially in early stages, detecting them could provide a critical early warning.
The Science Behind the Breakthrough
Scientists employ multi-step algorithms and cross-checks to analyze blood samples, searching for modifications in DNA patterns associated with tumors. This technique forms the basis of a Multi-Cancer Early Detection (MCED) test, designed to identify cancer-specific genetic changes in the blood.
The research team analyzed blood samples from 52 individuals:
- 26 people later diagnosed with cancer within six months of sample collection.
- 26 people who remained cancer-free.
The MCED test flagged eight cancer cases, a 31% detection rate, before any formal diagnosis or visible symptoms.
Promising Results and Key Challenges
The study's most striking finding was the analysis of older blood samples. Six of the eight detected individuals had samples available from 3.1 to 3.5 years before their diagnosis. Cancer signals were present in four of these six samples, albeit at levels significantly lower than the current test threshold. This indicates that tumors release DNA into the bloodstream well before symptoms manifest.

While promising, these results underscore the need to improve the test's sensitivity. Early-stage cancer is characterized by lower ctDNA levels, making detection challenging.
"This study shows the promise of MCED tests in detecting cancers very early," says Dr. Bert Vogelstein, a senior cancer researcher. "But it also sets the benchmark sensitivities required for these tests to succeed."
The Path Forward
Moving this technology from the lab to clinical practice requires rigorous clinical trials to validate its reliability and safety. Regulatory approvals are also necessary before widespread adoption. Furthermore, establishing appropriate clinical follow-up procedures after a positive test result is crucial. This may include further scans, biopsies, or even preventive treatments.
Despite these hurdles, this research signifies a significant and hopeful advancement in cancer diagnostics. Paired with ongoing improvements in treatment, particularly therapies targeting multiple cancer types, the future promises improved survival rates and a revolutionary approach to cancer screening and treatment.
Newer articles
Older articles
-
 Google CEO Sundar Pichai’s top 5 work tips to instantly boost productivity and leadership skill
Google CEO Sundar Pichai’s top 5 work tips to instantly boost productivity and leadership skill
-
 This new AI tool can help you book train tickets, get refunds and check details on IRCTC website and app
This new AI tool can help you book train tickets, get refunds and check details on IRCTC website and app
-
 How do graphic designers convert JPG to PDF (Portable Document Format)?
How do graphic designers convert JPG to PDF (Portable Document Format)?
-
 Priyanka Chopra reveals daughter Malti Marie has started school in New York: 'Her schedule is even crazier than mine'
Priyanka Chopra reveals daughter Malti Marie has started school in New York: 'Her schedule is even crazier than mine'
-
 Microsoft plans to take on iPhone and Android smartphones with this new device
Microsoft plans to take on iPhone and Android smartphones with this new device
-
 Mumbai’s Ravindra Sante to lead India’s mixed disability team on England tour
Mumbai’s Ravindra Sante to lead India’s mixed disability team on England tour
-
 Twitter bans over 5 lakh accounts in India, here's why
Twitter bans over 5 lakh accounts in India, here's why
-
 NASA astronauts prepare ‘space sushi’ aboard the ISS in zero gravity during a heartwarming crew celebration
NASA astronauts prepare ‘space sushi’ aboard the ISS in zero gravity during a heartwarming crew celebration
-
 Elon Musk’s AI company will make Grok chatbot more accessible, here’s how
Elon Musk’s AI company will make Grok chatbot more accessible, here’s how
-
 iQoo Z9 Turbo new leak reveals key specifications: All the details
iQoo Z9 Turbo new leak reveals key specifications: All the details